2.a. Contact lens – Induced papillary conjunctivitis (CLAPC):
CLAPC is Inflammation of the upper tarsal conjunctiva, often due to mechanical and immunological responses to contact lenses and their deposits, can cause hyperemia and roughness of the conjunctival surface. This condition, characterized by the enlargement of papillae and a cobblestone appearance of the upper tarsal conjunctiva, is more common with reusable contact lenses compared to disposable ones. High modulus silicone hydrogel lenses, in particular, increase frictional irritation, elevating the risk of this inflammation.
Signs:
- Deposits on the surface of contact lenses are one of the most common signs of Contact Lens-Induced Papillary Conjunctivitis (CLAPC).
- The condition may be localized or generalized.
- Enlargement of papillae greater than 0.30 mm, palpebral hyperemia, and mucus secretion. Giant papillae, with a diameter exceeding 1 mm, may also be present.
- Papillae are typically seen in the upper eyelid, requiring the upper lid to be everted for viewing.
- Fluorescein staining helps examine changes, as it pools around the valleys of these enlargements and stains the apices of the papillae when the disease is active.
- In chronic cases, the apices may appear whitish due to scarring.
- Conjunctival edema may also be present.
Symptoms:
- Asymptomatic in the early stages.
- Itching.
- Foreign body sensation.
- Lens intolerance.
- Reduced wearing time due to increased lens awareness.
Aetiology:
- The condition is considered multifactorial.
- Lens front surface deposits cause mechanical irritation. Deposits can act as antigenic components, triggering an ocular immune response.
- An immunological response to bio-deposits that accumulate on contact lenses.
Management:
- The prognosis for recovery from CLAPC is good after the removal of lenses and cessation of wear, with symptoms typically disappearing within 5 days to 2 weeks.
- Pharmacological therapy includes antihistamines combined with a mast cell stabilizer. Topical steroids are reserved as a last resort.
- Optimized lens care and maintenance.
- Daily disposable or frequently replaced contact lenses.
2.b. Meibomian Gland Dysfunction:
Meibomian gland dysfunction (MGD) is recognized as the primary cause of dry eye syndrome. This chronic condition affects the meibomian glands and is typically marked by blockage of the terminal ducts and/or qualitative or quantitative changes in glandular secretions. MGD can lead to alterations in the tear film, eye irritation, visible inflammation, and ocular surface disease. Contact lens wearers may experience mechanical trauma to the eyelid margin due to continuous rubbing, which can desquamate the epithelium and block the meibomian duct orifices, potentially resulting in gland atrophy (Irfan, S. 2019). Additionally, wearing contact lenses has been linked to a reduction in the number of functional meibomian glands (Arita et al. 2009).
Signs:
- Typically affects both eyes (bilateral)
- Roughness of the eyelid margin
- Cloudy, creamy yellow secretions upon gland expression
- Frothing or foaming at the lower tear meniscus
- Corneal staining
- Reduced tear break-up time
Symptoms:
- Sensation of dry eyes
- Burning sensation
- Intolerance to contact lenses
Aetiology
The primary cause is the blockage of gland orifices due to keratinization of the meibomian gland epithelia, which hinders or completely stops the outflow of meibomian oils.
Management
- Hot Compresses: Applying warmth to the eyelids to soften glandular secretions.
- Mechanical Expression: Manually expressing the glands to clear blockages.
- Lid Scrubs and Massage: Cleaning the eyelids with baby shampoo or specialized lid care products. The most common and cost-effective method involves using cotton buds and baby shampoo.
- Antibiotics: Prescribing antibiotics to manage infection.
- Artificial Tears: Using artificial tears to relieve dryness.
- Oral Tetracycline: Administering oral tetracycline for its anti-inflammatory properties.
- Antibiotic and Steroid Treatments: Combining antibiotics with steroids to reduce inflammation and infection.
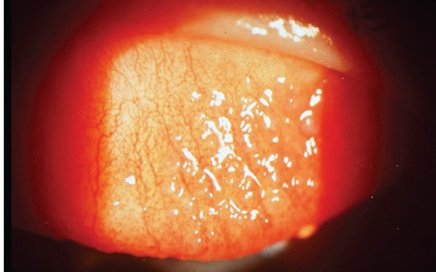
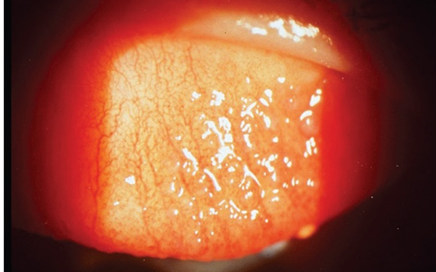
2.c. CLARE (Contact lens-induced Acute Red Eye)
Contact Lens-Induced Acute Red Eye (CLARE) is characterized by an acute, non-infectious infiltrative keratitis, presenting with sharp ocular pain and pronounced conjunctival hyperemia (Bruce et al., 2013). Often referred to as “3am syndrome” due to its frequent early morning onset, CLARE is more commonly observed in females and primarily associated with extended wear contact lenses.
Signs:
- Pronounced ocular redness affecting limbal and conjunctival vessels
- Presence of stromal infiltrates
- Minimal or no staining
Symptoms:
- Painful, red eyes
- Sensitivity to light (photophobia)
- Excessive tearing (lacrimation)
- General irritation
- Sensation of a foreign body in the eye
Predisposing Factors
- Prolonged contact lens wear
- Tight-fitting or high-water-content lenses
- Noncompliance with lens replacement schedules
- Improper use of contact lens solutions
- Poor contact lens hygiene practices
- Recent history of upper respiratory tract infections.
Aetiology
CLARE is triggered by toxins from Gram-negative bacteria, which induce an immune or inflammatory response in the eye during sleep. Overnight lens wear can trap debris and deposits, potentially initiating this inflammatory response. Additionally, sensitivity to contact lens care products may contribute to the condition.
Management
- Immediate Discontinuation: Cease contact lens wear immediately upon onset of symptoms.
- Monitoring: Regular monitoring is necessary, though medical intervention is usually not required.
- To relieve the pain use of artificial tears & cold compresses.
- Post-Recovery Refitting: Once recovery is confirmed, patients should be refitted with daily wear lenses to prevent recurrence.
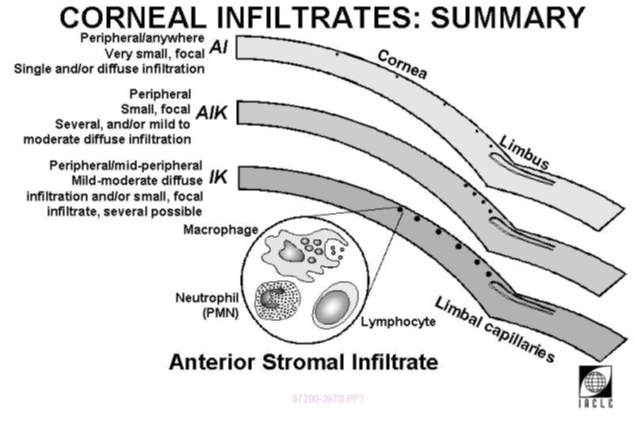
2.d. Corneal infiltrates:
Corneal infiltrate is relatively common condition induced by the contact lens (Stapleton et al. 2007). Corneal infiltrates are the collection of inflammatory cells (Macrophage, Neutrophil and lymphocytes) in the corneal tissue due to inflammation. three main corneal infiltrate condition are present depending on the symptoms and location of the infiltrate present.
- IK – Infiltrative keratitis
- AIK – Asymptomatic Infiltrative keratitis
- AI – Asymptomatic Infiltrates
Aetiology:
Inflammatory responses in the cornea can be induced by various stimuli associated with contact lens use, including mechanical, hypoxic, and toxic factors. These responses lead to the infiltration of inflammatory cells into the cornea (Robboy et al., 2003).
Management:
- Discontinue the lens wear and monitor till recovery.
- Find the root cause to reduce the risk of reoccurrence.
2.e. Microbial keratitis
Microbial keratitis is a serious and potentially dangerous condition characterized by inflammation of the cornea. Factors such as chronic ocular surface disease, corneal trauma, ocular surgery, and contact lens wear can make the cornea more susceptible to infection. Microbial keratitis is one of the most challenging complications associated with contact lens use. MK is caused by bacteria, fungi, or parasites. Bacterial keratitis is the leading cause of microbial keratitis, responsible for approximately 90% of cases (Musa et al., 2010). Most bacterial ulcers related to contact lens use are caused by Pseudomonas (Green et al., 2008). Acanthamoeba associated MK, while relatively rare but stands out as one of the most serious and challenging to treat (Zimmerman et al., 2016).
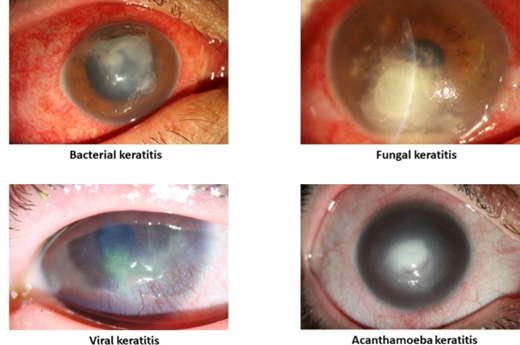
Signs:
- Conjunctival inflammation and discharge
- Corneal epithelial defects (confirmed with fluorescein)
- Corneal inflammatory infiltrate
- Thinning or perforation of the cornea
- Hypopyon
- Lid Oedema
- Earliest sign of acanthamoeba keratitis a molten dendritic epithelial defect which later on form a ring
Symptoms:
- Redness of the eye
- Pain
- Blurring of vision
- Photophobia
- Watering or discharge from the eye.
Aetiology:
Microbial keratitis associated with contact lens use is typically caused by bacteria, while infections from virus, fungi or Acanthamoeba are much less common. Microbial keratitis in contact lens wearers is often linked to noncompliant or unhygienic practices. These risky behaviours, including extended wear of lenses, poor hygiene of storage cases, infrequent case replacement, smoking, lack of hand washing, and purchasing lenses online, are all modifiable factors (Zimmerman et al., 2016).
Management:
- Discontinue wearing contact lens
- Culture swabs from the eye lesion, cornea and contact lens cases.
- Antibiotics based on culture results and monitor until resolved.
- No steroid until infection is controlled.
- Switch to Daily disposable.
2.f. Contact lens Induced peripheral ulcer (CLPU)
Contact lens-associated microbial keratitis can be ulcerative or non-ulcerative forms. A positive culture for bacteria, viruses, fungi, or amoebae confirms an infectious (microbial) keratitis. However, CLPU is a negative culture, does not rule out infection, as the microorganisms are not detected in the tissue (Aasuri et al., 2003). Ulcer is usually located peripherally.
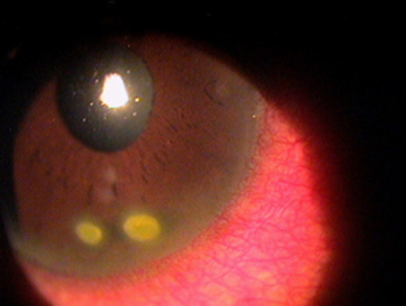
Sign:
- Redness
- Lacrimation
- Corneal epithelial defects (confirmed with fluorescein)
- Negative culture
Symptoms:
- Asymptomatic to severe pain
- Foreign body sensation
- Photophobia
- Decreased corneal sensitivity
Aetiology:
- Endotoxin from gram positive bacteria (Staphylococcus sp, Corynebacterium sp)
Management:
- Discontinue wearing lens and monitor
- Prophylaxis antibiotic for preventive measure
Reference:
- Holden BA, Mertz GW. (1984). Critical oxygen levels to avoid corneal edema for daily and extended wear contact lenses. Invest Ophthalmol.Vis.Sci, 25, 1161-1167.
- Harvitt, D. M., & Bonanno, J. A. (1999). Re-evaluation of the oxygen diffusion model for predicting minimum contact lens Dk/t values needed to avoid corneal anoxia. Optometry and vision science : official publication of the American Academy of Optometry, 76(10), 712–719. https://doi.org/10.1097/00006324-199910000-00023
- Bruce, A. S., & Brennan, N. A. (1990). Corneal pathophysiology with contact lens wear. Survey of Ophthalmology, 35(1), 25–58. https://doi.org/10.1016/0039-6257(90)90046-x
- NicholsJJ. Contact lenses 2008. Contact Lens Spectrum 2009;24:24‑32
- CL-associated papillary conjunctivitis (CLAPC), Giant papillary conjunctivitis (GPC). (n.d.). College of Optometrists. https://www.college-optometrists.org/clinical-guidance/clinical-management-guidelines/cl-associatedpapillaryconjunctivitis_clapc_giantpa
- Tan, M. E., Demirci, G., Pearce, D., Jalbert, I., Sankaridurg, P., & Willcox, M. D. P. (2002). Contact Lens-Induced papillary conjunctivitis is associated with increased albumin deposits on extended wear hydrogel lenses. In Advances in experimental medicine and biology (pp. 951–955). https://doi.org/10.1007/978-1-4615-0717-8_134
- Raju K. Contact Lens Induced Papillary Conjunctivitis- Review and A Case Report from Nepal. J Ophthalmol 2019, 4(1): 000172.
- Irfan, S. (2019). Meibomian gland dysfunction. Pakistan Journal of Ophthalmology, 35(1). https://doi.org/10.36351/pjo.v35i1.866.
- Arita, R., Itoh, K., Inoue, K. et al. (2009) Contact lens wear is associated with decrease of meibomian glands. Ophthalmology, 116, 379–384.
- Bruce, A. S., & Nguyen, L. M. (2013). Acute red eye (non‐ulcerative keratitis) associated with mini‐scleral contact lens wear for keratoconus. Clinical and Experimental Optometry, 96(2), 245–248. https://doi.org/10.1111/cxo.12033
- STAPLETON, F., KEAY, L., JALBERT, I., & COLE, N. (2007). The epidemiology of contact lens related infiltrates. Optometry and Vision Science, 84(4), 257–272. https://doi.org/10.1097/opx.0b013e3180485d5f
- Robboy, M. W., Comstock, T. L., & Kalsow, C. M. (2003). Contact lens-associated corneal infiltrates. Eye & contact lens, 29(3), 146–154. https://doi.org/10.1097/01.ICL.0000072830.41886.1E
- Musa, F., Tailor, R., Gao, A., Hutley, E., Rauz, S., & Scott, R. a. H. (2010). Contact lens-related microbial keratitis in deployed British military personnel. British Journal of Ophthalmology, 94(8), 988–993. https://doi.org/10.1136/bjo.2009.161430
- Green M, Apel A, Stapleton F (2008). Risk factors and causative organisms in microbial keratitis. Cornea. 27(1):22–27.
- Zimmerman, A., Nixon, A., & Rueff, E. (2016). Contact lens associated microbial keratitis: practical considerations for the optometrist. Clinical Optometry, 1. https://doi.org/10.2147/opto.s66424
- Aasuri, M. K., Venkata, N. and Kumar, V. M. (2003) Differential diagnosis of microbial keratitis and contact lens induced peripheral ulcer. Eye Contact Lens, 29(1 Suppl), S60–62.
- Holden, B. A., Stephenson, A., Stretton, S., Sankaridurg, P. R., O’Hare, N., Jalbert, I., & Sweeney, D. F. (2001). Superior Epithelial Arcuate Lesions with Soft Contact Lens Wear. Optometry and Vision Science, 78(1), 9–12. https://doi.org/10.1097/00006324-200101010-00008
- Van Der Worp, E. (2021). Corneal desiccation in rigid gas permeable contact lens wear : time to deal with 3- and 9-o clock staining. https://doi.org/10.26481/dis.20081128ew
- Van der worp E, de Brabander J, Swarbrick HA, Hendrikse F (2009). Evaluation of signs and symptoms in 3 and 9 o’clock staining. Optom Vis Sci. 86(3):260-5.
Lecturer (Nethradhama School of Optometry)
Moptom